Introduction
Rare renal disorders – conditions affecting the kidneys that occur in a small percentage of the population – pose significant challenges for patients, clinicians, and researchers. Unlike common kidney diseases such as diabetic nephropathy or hypertensive nephrosclerosis, rare renal disorders often have complex genetic underpinnings, heterogeneous clinical manifestations, and limited treatment options. However, recent advances in molecular medicine, targeted therapies, and precision medicine are transforming the therapeutic landscape, offering new hope for patients living with these debilitating conditions.
Definition
Rare Renal Disorders Therapeutics refers to the development and clinical use of specialized drugs, biologics, gene therapies, and supportive treatment strategies designed to diagnose, manage, or cure uncommon kidney diseases, often with genetic, metabolic, or autoimmune origins. These therapeutics aim to address limited treatment options by targeting specific disease mechanisms, improving kidney function, slowing disease progression, and enhancing quality of life for patients affected by rare renal conditions.
Understanding Rare Renal Disorders
Before discussing therapeutics, it’s important to understand what constitutes a rare renal disorder. A disease is typically classified as “rare” when it affects fewer than 1 in 2,000 individuals, although the definition may vary slightly by region. Rare renal disorders encompass a broad spectrum, including:
- Genetic and hereditary diseases such as Alport syndrome, Fabry disease, and polycystic kidney disease (PKD) variants.
- Congenital anomalies of the kidney and urinary tract (CAKUT).
- Metabolic disorders with renal involvement like cystinosis and primary hyperoxaluria.
- Rare glomerulopathies such as C3 glomerulopathy and fibrillary glomerulonephritis.
- Tubulopathies including Bartter and Gitelman syndromes.
The rarity, variability, and often multisystemic nature of these diseases make diagnosis and management particularly challenging. Traditional therapies have largely been supportive (e.g., blood pressure control, dialysis, or transplantation) rather than curative.
Challenges in Therapeutics Development
Developing therapies for rare renal diseases carries inherent difficulties:
Small Patient Populations:
Clinical trials typically require large sample sizes to show statistically significant outcomes. For rare diseases, enrolling enough patients is difficult, lengthening trial timelines and increasing costs.
Heterogeneous Clinical Presentation:
A single genetic mutation can produce different symptoms across patients, complicating trial design and endpoint selection.
Lack of Disease Understanding:
Many rare renal disorders were historically understudied. Without clear pathophysiological insights, identifying drug targets is challenging.
Regulatory Hurdles:
Regulatory pathways for rare disease therapies are improving but still demand rigorous safety and efficacy evidence. Even with incentives such as orphan drug designation, the pathway to approval can be long.
Despite these obstacles, scientific innovation and supportive policies have accelerated progress – ushering in a new era of targeted and transformative therapies.
Breakthroughs in Rare Renal Disorder Therapeutics
1. Gene-Targeted and Precision Therapies
One of the most promising areas of development involves targeted therapies based on the genetic drivers of disease.
- RNA-Based Therapies
RNA interference (RNAi) and antisense oligonucleotides (ASOs) have emerged as effective tools to silence disease-causing genes. For example, in primary hyperoxaluria type 1 (PH1), RNAi agents like lumasiran reduce oxalate production by targeting key enzymes in glyoxylate metabolism, slowing disease progression and reducing renal damage.
- Gene Editing
CRISPR-Cas9 and other gene-editing technologies hold promise for correcting pathogenic mutations at their source. While still largely in preclinical or early clinical stages for renal disorders, gene editing could eventually offer one-time curative treatments for monogenic kidney diseases like Alport syndrome.
2. Small Molecule Targeted Therapies
Small molecule drugs remain a cornerstone of treatment, particularly when they can modulate disease pathways directly.
- Tolvaptan in Autosomal Dominant Polycystic Kidney Disease (ADPKD): This vasopressin receptor antagonist slows cyst growth and kidney function decline and represents a major advance for a disease that was previously managed only with supportive care.
- Migalastat for Fabry Disease: A pharmacological chaperone that stabilizes the defective alpha-galactosidase A enzyme in amenable mutations, improving kidney function and reducing the accumulation of toxic substrates.
- SGLT2 Inhibitors: Originally developed for diabetes, these drugs also offer kidney protection in certain rare renal conditions due to their hemodynamic and metabolic effects.
3. Enzyme Replacement and Substrate Reduction Therapies
For diseases caused by enzyme deficiencies or metabolic accumulation, replacement or reduction strategies are effective:
- Cystinosis: Cysteamine, a cystine-depleting agent, slows kidney deterioration and improves outcomes.
- Fabry Disease: Enzyme replacement therapy (ERT) with recombinant alpha-galactosidase reduces substrate buildup and delays renal and cardiac complications.
4. Cell and Regenerative Therapies
Stem cell and regenerative medicine approaches aim to repair or replace damaged renal tissue.
- Mesenchymal Stem Cells (MSCs): Early research suggests MSCs may reduce inflammation and promote repair in certain kidney disorders, though clinical evidence remains preliminary.
- Bioengineered Kidneys: Long-term goals include growing functional kidney tissue for transplantation – a transformative solution for end-stage renal disease arising from rare disorders.
Regulatory and Supportive Frameworks
The evolving therapeutic landscape for rare renal diseases is supported by regulatory incentives and collaborative research efforts:
- Orphan Drug Designation: Agencies like the FDA and EMA offer incentives such as tax credits, market exclusivity, and fee waivers to encourage development of therapies for rare diseases.
- Natural History Studies and Registries: These generate critical data on disease progression, assisting trial design and endpoint selection.
- Patient Advocacy Groups: Organizations such as the Alport Foundation, PKD Foundation, and Rare Kidney Stone Consortium are instrumental in funding research, increasing awareness, and patient recruitment for studies.
Real-World Impact: Patients and Providers
Advances in therapeutics are not just scientific milestones — they translate into real benefits for patients:
- Improved Quality of Life: New treatments can slow disease progression, reduce symptoms, and delay or avoid dialysis and transplantation.
- Personalized Care: Genetic testing and precision medicine allow clinicians to tailor therapies based on individual molecular profiles.
- Empowered Patients: Better understanding of their conditions enables patients to participate actively in treatment decisions and clinical trials.
However, challenges remain: access and affordability of novel therapies can be barriers, particularly in low-resource settings. Ongoing efforts to expand global access and equitable care are critical.
Future Directions
The future of rare renal disorder therapeutics is bright, driven by scientific innovation and collaborative ecosystems:
- Expanded Genetic Therapies: Beyond single-gene disorders, polygenic and complex mechanisms may become targets for combination genetic and pharmacologic strategies.
- Biomarkers and Digital Health: Improved biomarkers will enable earlier diagnosis, real-time monitoring, and adaptive clinical trials. Wearable and digital tools may further personalize care.
- Global Research Networks: International collaboration will be essential to pool data, share resources, and accelerate drug development.
- Health Policy and Economics: Value-based pricing, outcome-based reimbursement, and patient assistance programs will play roles in ensuring novel therapies reach those who need them.
Growth Rate of Rare Renal Disorders Therapeutics Market
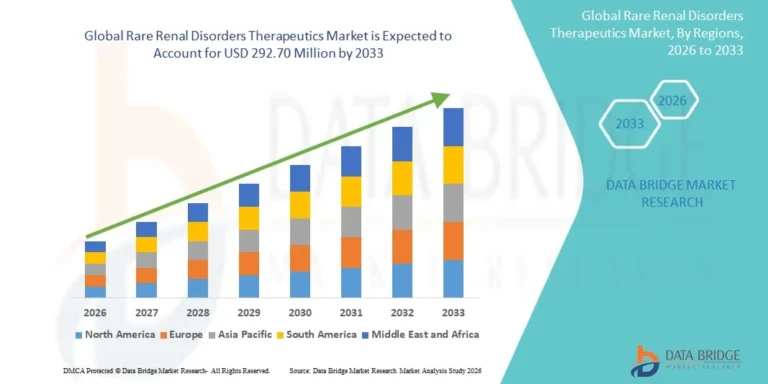
According to Data Bridge Market Research, the rare renal disorders therapeutics market was estimated to be worth USD 128.70 million in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 10.80% to reach USD 292.70 million by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-rare-renal-disorders-therapeutics-market
Conclusion
Rare renal disorders, once relegated to symptomatic management and supportive care, are entering a new chapter of therapeutic possibility. Through breakthroughs in genetics, targeted therapies, and collaborative research, the focus is shifting toward precision medicine and disease-modifying treatments. While challenges — scientific, economic, and logistical — remain, the momentum of discovery offers hope to patients, families, and clinicians confronting these complex conditions.